Engineering Information on Stainless Steel Cables
PYTHON/INOX/Stainless Steel
Introduction
Chromium, as an alloy element, is primarily responsible for the outstanding corrosion resistance properties exhibited by stainless steel and stainless steel products. This element is characterized by is passivity, that is, its ability to form thin, oxidization-protective layers. These excellent corrosion resistance properties are transferred to the steel if chromium is present as the alloying metal at concentrations of >12 %. The structure of such purely Cr-alloyed steel grades is ferritic.
A greater corrosion resistance can be achieved by the addition of nickel at concentrations in excess of 8%. Since, at these concentrations, nickel already acts to stabilize austenite, Cr/Ni-alloyed steel grades that have undergone corresponding annealing and quenching treatment exhibit a purely austenite structure even at room temperature. This is the reason for the high cold-work hardness and nonmagnetic behavior (paramagnetic behavior) of these steels.
A further improvement in corrosion resistance can be achieved by the addition of 2.0 -2.5% of molybdenum while simultaneously increasing the nickel content. Steel grades produced in this manner are characterized by their particularly high resistance to pitting.
When subjected to temperatures in excess of 300° C (570˚F), the precipitation of iron/chromium carbides at the grain boundaries increases the risk of inter-crystalline corrosion. Steel grades that have been made resistant to such stresses by the addi-tion of carbide-stabilizing elements such as titanium, niobium, and tantalum are able to avoid this thermal risk, particularly during welding.
Heat-resistant material No. 4841 (AISI 314) represents a further development in stainless steels. In such steels, the high affinity of chromium with respect to oxygen in the formation of protective layers that prevent an oxidization of the material is also utilized. These properties are then improved by increasing the chromium content up to 25 %, together with a simultaneous increase in the silicon content or by the addi-tion of small amounts of aluminum. In addition, a significant increase in the thermal strength and workability of these steel grades was achieved by the addition of nickel up to concentrations of 20%.
Stainless steel wire cables are employed wherever there is a need for a high degree of corrosion resistance in conjunction with high strength, in other words, wherever cables made of plastic, of nonferrous metals, or steel cables with corro-sion-protecting coatings of zinc, copper, tin, brass, or plastic are inadequate.
Because of their outstanding physical and chemical properties, their application is currently increasing rapidly in nearly every area of the chemical and medical industry, in ship and boatbuilding, in aeronautics, as well as in reactor construction, in architecture and facade decoration, in machinery and equipment construction, and in underground cable engineering.
Detailed information regarding the most practical choice of cable for your particular needs are optimally obtained through close consultation with WDI or Unirope. Please provide information concerning the operating conditions under which your cable will be employed (e.g., type, concentration, and temperature of any acids), make your requirements known.
Despite the corrosion resistance and strength of stainless steels the corrosion problems described below may still arise under certain conditions.
Contact corrosion can arise as a result of contact between several metals with differing electrode potentials in the presence of an electrolyte. In the passive state, the potential of austenitic chromium/nickel steels exhibits a higher positive value. Stainless steel wires made of material No. 4301 (AISI 302/304) are generally not affected by contact with other materials such as copper, bronze, or brass, since they assert themselves as cathodes with respect to these materials. However, should a reducing electrolyte abolish the passivity, corrosion will generally occur.
Pitting represents a localized corrosion attack, sometimes limited to the smallest surface areas, but with very deep-acting effects. It is the result of the formation of perforation elements. Corrosion of this nature occurs primarily in the presence of halogen compounds. Encrustations also promote the formation of perforation elements. In many cases, damage can be avoided by simply keeping the surface at risk clean. No. 4401 (AISI 316) grades alloyed with molybdenum exhibit less pitting than the more common chommium-nickel steels with material No. 4301 (AISI 302/304).
In the presence of specific chemical agents, stress corrosion cracking can occur in austenitic chromium/nickel steels subject to external or internal stresses (residual stresses). Such agents primarily include solutions containing chloride, moist salts and other salt encrustations, and caustic and pickling solutions containing chlorine. Stress corrosion cracking may also occur under certain circumstances where foreign corrosion deposits exist. The cracks exhibit a great deal of branching and occur at both the inter- as well as the intra-crystalline level. Cracks generally form perpendicular to the direction of the stress.
In the case of unstabilized ferritic and austenitic chromium and chromium/nickel steels, annealing in the sensitization range can result in a tendency towards grain disintegration. For ferritic steels, this temperature range lies at approx.1,000° C (1,830°F), while, for austenitic steels, it lies between 450° and 900° C (840˚ and 1650˚F). Annealing leads to a precipitation of iron/chromium carbides at the grain boundaries, in turn resulting in a certain degree of chromium deficit in the region immediately surrounding the precipitation. In such cases, the grain boundaries then react actively to chemical action. The corrosion spreads very rapidly along the grain boundary and results in the separation of grains and the disintegration of the component. The sensitization temperatures mentioned above occur primarily during welding, in the area around the weld seam. The unfavorable precipitation can be reversed by appropriate tempering afterwelding or can be avoided altogether by the employment of grades stabilized with titanium or tantalum/niobium or which exhibit a very low carbon content for applications where welding is required. The addition of titanium, niobium, and tantalum binds carbon into stable carbides. Since stainless steel cables and wires are generally not exposed to the sensitization temperatures indicated above, corrosion resulting from grain disintegration is of no direct significance to them.
Rope fittings connections made of heavy metal alloys (e.g., bronze, copper or brass) do not generally produce areas of contact corrosion when they are used on stainless steel cables.. However, only stainless steel connecting elements are truly ideal. Just as is the case with any steel cables, stainless steel cables can also be spliced. For screw type end connections formed by plugging, contact corrosion is generally not anticipated at the plug sections of the cables with antifriction metal. A clean surface, free of oxides is the prerequisite for the passivity of stainless steels. Matte-finished surfaces also ensure the passive state of stainless steels.
Lubrication merely serves to reduce the internal cable friction. This makes the cable more resistant to mechanical stresses.
Cables fashioned from cold-hardened, austenitic steel wires made of material no. 4301 and 4401 (AISI 302/304 and 316) are slightly ferromagnetic. Where physical reasons permit very low permeability values (= 1. 10 G/Oe), grades fashioned from material no. 3956 (AISI 320) should be employed.
Material no. 4841 (AISI 314) can be used for temperature ranges up to 1,200°C. However, this material is sensitive to gases containing sulfur. Material no. 4301 or 4401 (AISI 302/304 or 316) is adequate for temperatures between 250° – 300°C (480°F to 570°F).
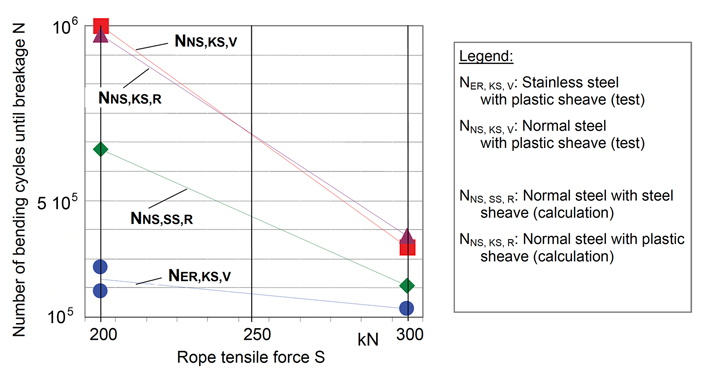
Only very little research has been carried out on the bending fatigue of stainless steel ropes running over sheaves. Preliminary test results under fluctuating tension and bending stresses showed the lifetime to be lower than with traditional carbon steel wire ropes by a factor 3 to 8.
To compensate for the reduced bending fatigue life sheave D/d ratios may need to increase by a factor of 3 to 5 and the rope tension be reduced by about half compared to ropes made from carbon steel.
(Vogel/Schoenherr/Wehking, OIPEEC 2007)